Background
Patients (pts) with Philadelphia chromosome-negative (Ph-neg) myeloproliferative neoplasms (MPNs) that progress to an accelerated phase (AP) or blast phase (BP) have a median overall survival (mOS) of less than 1 year (Odenike, Blood 2018; Tam et al, JCO 2009). Allogeneic hematopoietic stem cell transplant (allo-HCT) is the only curative approach for MPN-AP/BP, but mOS remains about a year for pts with MPN-BP that undergo allo-HCT (2005-2019) (Orti et al, AJH 2023). We previously reported on outcomes of pts with MPN-AP/BP treated in the current era of myeloid therapies (2017 onward) and found a mOS of 0.73 years in a multi-center cohort of 80 pts (Patel et al, ASH 2022). We aim to characterize treatment patterns and outcomes of pts with MPN-AP/BP that underwent allo-HCT via a multi-center retrospective analysis.
Methods
Retrospective chart review was performed at 8 institutions to identify pts with pathology-confirmed Ph-neg MPN-AP/BP diagnosed in 2017 or later. All patients that met inclusion criteria were included. Response was assessed using 2017 European Leukemia Net AML (2017 ELN) criteria (Dohner et al, Blood 2017). OS from diagnosis of MPN-AP/BP and from time of allo-HCT was calculated utilizing Kaplan-Meier analysis.
Results
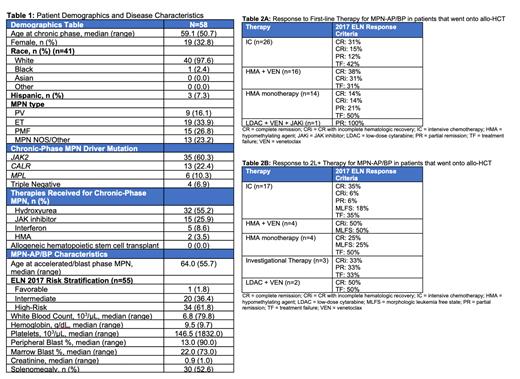
The full MPN-AP/BP cohort consists of 180 pts. This analysis includes 58 pts that underwent allo-HCT for MPN-AP/BP across 8 institutions. Median age at MPN-AP/BP diagnosiswas 64 years. The most common therapies for chronic-phase MPN included hydroxyurea (55%), JAK inhibitor (JAKi) (26%), interferon (9%), and hypomethylating agent (HMA, 4%). No patient previously received allo-HCT for chronic-phase MPN. Driver mutations included JAK2 (60%), CALR (22%), MPL (10%), but some pts had triple-negative disease (7%). Sixty-two percent of pts had high-risk disease by 2017 ELN criteria. Forty-four pts had next-generation sequencing performed at time of MPN-AP/BP diagnosis. The most frequently co-occurring mutations detected at time of MPN-AP/BP diagnosis were ASXL1 (30%), SRSF2 (23%), RUNX1 (23%), TP53 (18%), TET2 (16%), IDH2 (11%), U2AF1 (7%), EZH2 (7%), and IDH1 (9%). Additional characteristics are in Table 1.
One pt proceeded directly to allo-HCT at time of MPN-AP/BP diagnosis. Frontline therapies (1L) for MPN-AP included intensive chemotherapy (IC) (n=26), hypomethylating agent (HMA) + venetoclax (VEN) (n=16), HMA monotherapy (n=14), and low-dose cytarabine (LDAC) + VEN + JAKi (n=1). Responses are summarized in Table 2A. Thirty-seven pts proceeded to allo-HCT after 1L therapy (17 after IC, 10 after HMA+VEN, 9 after HMA, 1 after LDAC+VEN+JAKi). Twenty pts proceeded to allo-HCT after 2 nd-line and beyond (2L+) therapies. Therapies utilized and responses are summarized in Table 2B.
Transplant-specific characteristics include 15 pts receiving myeloablative conditioning while 43 received reduced-intensity conditioning. Donor source was matched unrelated donor for 33 pts, haploidentical donor for 10 pts, matched related donor for 9 patients, mismatched unrelated donor for 5 patients, and cord blood for 1 patient. Twenty-two pts developed acute GVHD while 15 developed chronic GVHD. Amongst the 58 pts that underwent allo-HCT, 19 had relapse of disease and 8 patients received treatment after relapse.
Amongst the full cohort of 180 patients, mOS was 0.72 years from time of MPN-AP/BP diagnosis. The mOS for the 58 pts that underwent allo-HCT was 1.82 years from time of MPN-AP/BP diagnosis and 1.30 years from time of allo-HCT. Survival analysis by initial MPN-AP/BP therapy demonstrated a mOS from time of MPN-AP/BP diagnosis of 1.9 years for HMA-based therapy, 1.9 years for HMA + VEN, and 1.5 years for IC ( p=0.006). We also analyzed mOS by disease status prior to transplant. Thirty-four pts had a complete response (CR) or CR with incomplete count recovery (CRi) with mOS of 1.7 years from time of allo-HCT, 12 pts with a partial response (PR) or morphologic leukemia-free state (MLFS) had mOS of 1.8 years, and 11 pts with treatment failure (TF) had mOS of 0.47 years (p=0.82).
Conclusions
Our cohort of 180 pts with MPN-AP/BP treated in the current era of myeloid therapies had limited survival with a mOS of 0.72 years from MPN-AP/BP diagnosis. Amongst the 58 pts that underwent allo-HCT the mOS was 1.82 years with longer mOS seen in pts that received HMA or HMA + VEN as their 1L therapy when compared to IC. This underscores the need for novel management strategies even in pts eligible for allo-HCT.
OffLabel Disclosure:
Patel:Kronos Bio: Research Funding; AbbVie: Honoraria; BMS: Honoraria; Pfizer: Research Funding. Shallis:Rigel: Consultancy; Curio Science: Consultancy; Servier: Consultancy; Gilead Sciences: Consultancy; Bristol Myers Squibb: Consultancy. Chen:Rigel: Consultancy; Abbvie: Consultancy. Rampal:Celgene-BMS: Consultancy; Kartos: Consultancy; Zentalis: Consultancy; Karyopharm: Consultancy; Incyte: Research Funding; Constellation: Research Funding; Servier: Consultancy; Morphosys/Constellation: Consultancy; Sumitomo: Consultancy; Zentalis: Research Funding; CTI BioPharma Corp: Consultancy; Stemline: Research Funding; Ryvu: Research Funding; Dainippon: Consultancy; GSK-Sierra: Consultancy; Galecto: Consultancy; Pharmaessentia: Consultancy; Incyte: Consultancy. Bradley:NOVARTIS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron Corporation: Consultancy; Gilead: Membership on an entity's Board of Directors or advisory committees. Abaza:Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Rigel: Honoraria, Membership on an entity's Board of Directors or advisory committees; Biomea: Research Funding; Curis: Research Funding; Biosight: Research Funding; ALX Oncology: Research Funding. Garcia:Gilead: Consultancy; AstraZeneca: Research Funding; New Wave: Research Funding; AbbVie: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Astellas: Consultancy; Servier: Consultancy; Genentech: Consultancy, Research Funding; Pfizer: Research Funding; Prelude: Research Funding. Gupta:GSK: Other: Travel to EHA 2023 for invited talk at GSK sponsored MPN education session ; Novartis, BMS Celgene, SMP Oncology, AbbVie, Constellation Biopharma, Pfizer, GSK Pharma, CTI Biopharma: Consultancy; BMS, Celgene, Roche, Abb Vie, Pfizer, Sierra Oncology, CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Novartis, BMS Celgene, GSK: Honoraria; BMS Celgene, Roche, AbbVie, Pfizer, Sierra Oncology, CTI Biopharma, GSK: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Novartis, BMS Celgene, Sierra Oncology, AbbVie, Constellation Biopharma, Pfizer, GSK Pharma, CTI Biopharma: Consultancy. Pettit:Protagonist Therapeutics, Inc.: Consultancy, Research Funding; Merck: Research Funding, Speakers Bureau; AbbVie: Consultancy, Research Funding. Odenike:BMS/Celgene, Novartis, Rigel, Servier, Taiho ; DSMB-Kymera therapeutics: Membership on an entity's Board of Directors or advisory committees; ABBVIE, Astrazeneca, Agios, Aprea, Astex, BMS/Celgene, CTI, Daiichi, Incyte, Janssen, Kartos, Novartis, NS-Pharma and Oncotherapy Sciences: Research Funding.
outcomes of patients treated with investigational therapies while on trial are part of this retrospective analysis

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal